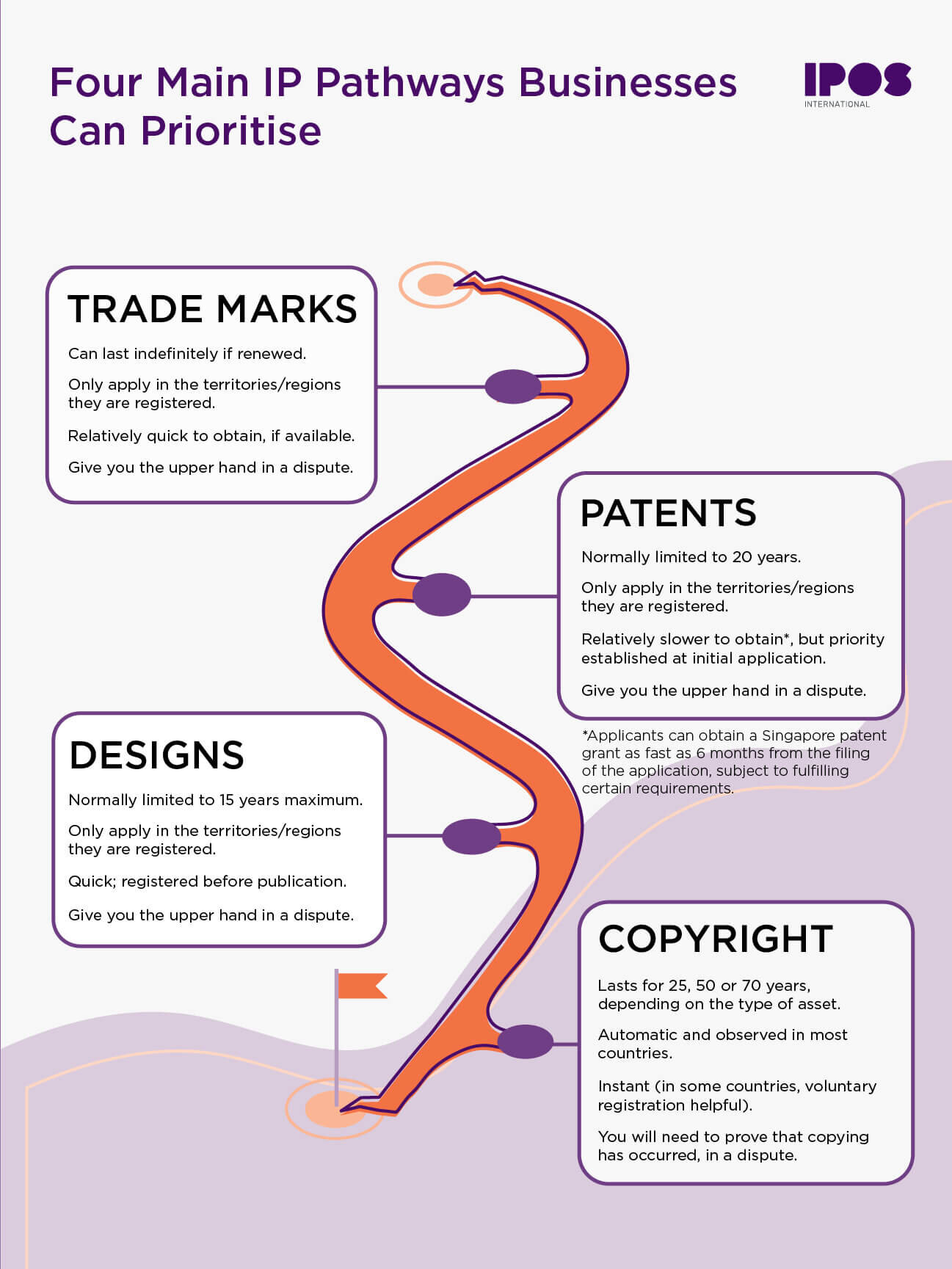
A Cure for Covid-19 - Patent Pending

This Op-Ed was first published in The Business Times.
Globally, scientists are rushing to find a cure for Covid-19. In the past five months, a record 2739 clinical trials have been registered with the World Health Organization.
In a global health crisis, the race to find a cure usually reflects two realities – first, the need to save lives as millions become infected; and second, the economic benefits such a cure would surely bring to its inventors, through patents.
Or would it?
Recent developments show that companies and governments are in fact working together to balance the forces between innovation, economic benefit and saving lives.
Teaching an old drug new tricks
Many companies are looking at “repurposing” existing antivirals to treat Covid-19.
Without compromising therapeutic efficacy, repurposed drugs have significant advantages in reducing development time and costs over new drugs. There may also be pre-established manufacturing processes and supply chains, allowing scalable production to meet surging demands.
Developing a brand-new drug can take up to 10 years. In this ongoing pandemic, savings in research and development can translate to lives saved.
Top drugs dominating global clinical trials include Chloroquine, Kaletra (Lopinavir/Ritonavir), and Remdesivir, favoured for their demonstrated efficacy in blocking coronaviruses that include the SARS and MERS.
Remdesivir was granted emergency approval in May by the United States’ Food and Drug Administration (FDA) as a treatment for severely ill Covid-19 patients.
Patents grant the right holders a monopoly over the production and sale of an invented drug. However, patents are territorial, meaning a patent granted in the US will not have legal effects outside of the US.
The patents for these drugs will have considerable influence on their price and availability, should any prove to be an effective treatment for Covid-19.
Read also: Patents and Trade Secrets: A Dual Strategy for Business Growth
The patents relating to Chloroquine in the US and China for therapeutic use in treating coronaviruses have lapsed, although no such patent application has been filed in Singapore.
Patents have been granted for the pharmaceutical formulations of Kaletra in the US, China, and Singapore, with their therapeutic use granted in the US. For Remdesivir, patents have been granted for its pharmaceutical formulations in the US, China, and Singapore. A patent for its therapeutic use in treating coronavirus has also been recently granted in the US, but this is still pending in the latter two countries.
Following the FDA’s emergency approval, the costs for Remdesivir are now expected to go up – though Gilead Sciences, Remdesivir’s manufacturer, has declined to say how it might charge for the drug.
Patents vs public health?
A global IP agreement known as the Trade-Related Aspects of Intellectual Property Rights (TRIPS), allows governments to invoke the compulsory use of patented technology under a public health emergency. Recently, Israel has invoked compulsory licensing of Kaletra.
Although compulsory licensing appears to offer a possible solution to counter the rising drug costs and increase drug supply, such a move may result in undesirable consequences.
A quality IP regime encourages innovation. It allows companies to recoup R&D investments, thereby creating economic benefits. It follows that an active stance of compulsory licensing may discourage companies from stretching limited resources to discover new cures, be it for Covid-19 or other emerging diseases.
Read also: Harnessing Data to Assess the Impact of a Viral Pandemic
The fact that Kaletra and Remdesivir can be readily repurposed for Covid-19 stem from patent-driven innovation. Therefore, stimulating innovation now is ever more critical not only to resolve the current pandemic but also to prepare us for future public health emergencies. Kaletra’s owner AbbVie has already announced it would dedicate its patent right on Kaletra to the public for Covid-19. Thus any country can purchase Kaletra generics to treat Covid-19.
This begs the question whether it is necessary to invoke compulsory licensing, if pharmaceutical companies are willing to give access to patented drugs in this pandemic.
Doing right with rights
Multiple companies from various industries have already pledged their IP rights free of charge under the “Open Covid Pledge”.
For example, IBM is granting free access to its huge patent portfolio while Medtronic has shared its ventilator patent.
Corporate social responsibility is evolving and has the immense potential to achieve both business and social goals in a mutually supportive manner.
Companies can also choose to help by voluntarily assigning existing and future IP rights relating to cures for Covid-19 to global patent pool endorsed by WHO, which provides access to every member state at a fair price.
Alternatively, companies can endorse multiple licensing arrangements to increase drug supply or lower drug pricing.
The quickening pace of sharing innovation signals that companies are in fact willing to contribute without governments resorting to compulsory licensing. And that innovation can still thrive whether it is driven by patents, or public health.
In these unprecedented times, we need to explore all creative avenues, cooperating with industries to balance the sustainability of innovation and the public health needs.